內容 Content
Abstract
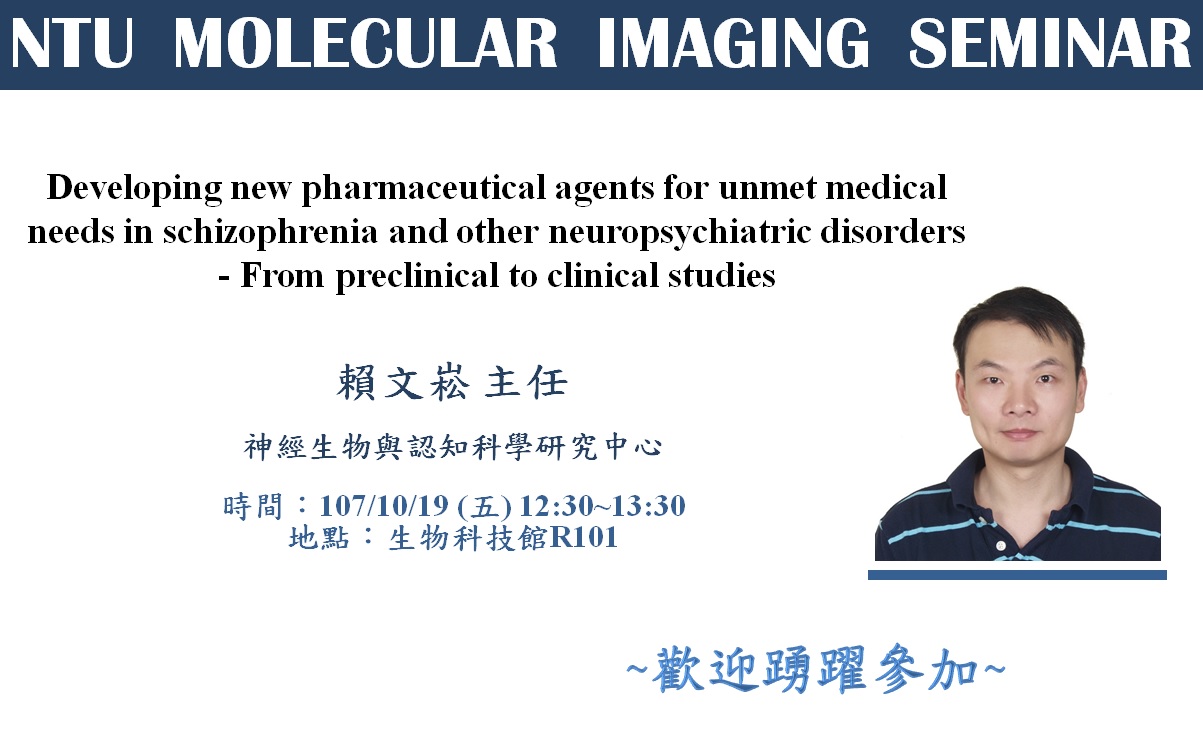
Schizophrenia, a severe mental illness, affects 1% of population worldwide, including Taiwan. Common symptoms associated with schizophrenia include positive, negative, cognitive, and mood-related symptoms. To date, there is no drug for the treatment of negative and cognitive symptoms. It has become an unmet medical need. According to Decision Resources, negative symptoms of schizophrenia will garner $1.5 billion in major-market sales, and the schizophrenia market will grow from $6.3 billion in 2012 to $8.0 billion in 2022. In recent years, the glutamate hypothesis of schizophrenia has attracted more attention. Excitatory neurotransmission mediated by N-methyl-D-aspartate (NMDA) receptor is primitive to and widely spread in mammalian CNS and NMDA receptor modulator has also been a popular target for many CNS diseases. Increasing evidence from genetic, clinical, and pharmacological studies further supports the NMDAR hypofunction hypothesis of schizophrenia. Notably, several NMDA-enhancing agents, especially through glycine modulatory site (GMS) of NMDAR, resulted in a significant reduction of psychotic and cognitive symptoms in some schizophrenic patients. Given that NMDAR-mediated signaling pathways has been implicated in cognitive/social functions and that GMS is a potential therapeutic target for enhancing activation of NMDARs, it is of great interest to investigate the effect of GMS modulators and their underlying mechanisms. Two examples from our team will be presented in this talk, including sarcosine and RS-D7. In this talk, our recent findings in sarcosine (i.e., a glycine transporter 1 inhibitor) and RS-D7 (a NCE) will be briefly introduced and revealed. A series of cell-based assays and mouse studies has been conducted and indicates their efficacy, therapeutic effects, and underlying mechanism. Taking advantage of RS-D7 can be converted through a market drug RS-D7pro, our team has further demonstrated RS-D7’s significant therapeutic potentials in negative/cognition symptoms of schizophrenia and other neuropsychiatric disorders in our proof-of-concept clinical studies. We hope we can build up further collaboration between our Neurobiology and Cognitive Science Center and MIC for developing new pharmaceutical agents to treat unmet medical needs in the near future.

 繁體中文
繁體中文 English (UK)
English (UK)